Chromatography
Simplifying Methods Transfer: Novel Tools for Replicating your Established Methods on the ACQUITY Arc System
Sep 17 2015
Retention shifts in methods transfer are commonly observed. Retention can be impacted by differences in system and pump characteristics such as dwell volume and mixing characteristics. Many laboratories require there be no adjustments to a method, particularly for validated methods. If adjustments are necessary, consideration is generally made to be in accordance with regulatory guidelines. With reference to dwell volume, chapter <621> of the USP monograph states "If adjustments are necessary, change in ... the duration of an initial isocratic hold (when prescribed), and/or dwell volume adjustments are allowed [1]". While adjustments for dwell volume can be manually entered into a gradient table, this approach requires calculations and manual changes to the gradient table; both of which require additional time and effort. Using a feature in the software to adjust the time between the start of the gradient and the point of injection, the duration of the gradient hold can be adjusted. This is accomplished without the need to make changes to the gradient table allowing systems - such as the ACQUITY ArcTM System- to mimic systems with different dwell volumes. By being able to enter the value directly in time, methods transfer can be streamlined by measuring the difference in retention time and adjusting the initial hold accordingly.
[for the full application note, please visit www.waters.com and search for 7200005469en, or here is the direct link http://www.waters.com/waters/library.htm?cid=511436&lid=134856832}
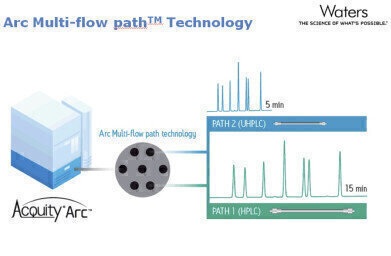
The ACQUITY Arc system minimises downtime and inefficiencies, allowing the seamless transfer of new technology into regulated environments. Enabled by the Arc multi-flow path™ technology, the ACQUITY Arc System can easily replicate methods developed on previous generations of LC instrumentation without alteration to the method. By simply selecting Path 1 or Path 2 in the system software, the user can emulate the dwell volume and mixing behaviour of their legacy HPLC systems in efforts to replicate their established HPLC methods without the need for revalidation.
The Arc multi-flow path technology was designed specifically to enable users to successfully transfer their established HPLC methods on to a modern LC platform, while providing them the flexibility to further improve their productivity when pairing the system with a more modern UHPLC column technology.
[For information on the ACQUITY Arc System, please visit www.waters.com/arc]
REFERENCES:
1. In United States Pharmacopeia and National Formulary (USP 37-NF 32 S2). ; United Book Press, Inc.: Baltimore, MD, 2014; Vol. , p 6376.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK