News & Views
Advanced Cell Diagnostics Receives ISO Certification
Jul 30 2014
Advanced Cell Diagnostics, a leader in the field of in situ nucleic acid detection for life science research and clinical diagnostics, has been granted ISO 13485:2003 certification for medical devices paving the way for the company to launch its CE marked diagnostic tests in Europe. The certification covers the design, development, production, and commercialisation of ACD’s proprietary RNAscope® product lines.
Said to address a long-standing technological gap in the analysis of RNA, the instrument has seen rapid adoption by both academic and industrial researchers since the beginning of its full commercialisation in 2011.
"ACD's vision is to make RNAscope the PCR equivalent for in situ nucleic acid detection and drive adoption of this technology in diagnostic tests," said Dr. Yuling Luo, President and CEO of ACD. "This ISO certification is the first step in our quality systems road map, which is designed to ensure that we supply products of the highest quality to our customers and provide a seamless path for our partners to translate scientific advances to patient benefits."
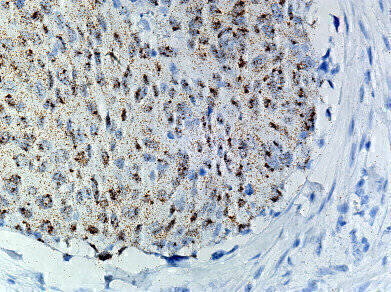
RNAscope detects and localise single RNA molecules in individual cells in intact tissues, enabling simultaneous molecular and histopathological analysis, the company said.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK
May 21 2024 Lagos, Nigeria











.jpg)