-
.jpg) Pic tured (l-r) Nir Erez, Dr. Noa Furth, Dr. Efrat Shema and Vadim Fedyuk
Pic tured (l-r) Nir Erez, Dr. Noa Furth, Dr. Efrat Shema and Vadim Fedyuk -
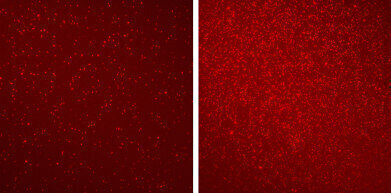 Different patterns of epigenetic markers revealed by EPINUC on blood nucleosomes (bright-red dots) of a healthy person (left) and a colorectal cancer patient (right)
Different patterns of epigenetic markers revealed by EPINUC on blood nucleosomes (bright-red dots) of a healthy person (left) and a colorectal cancer patient (right)
News & Views
Liquid Biopsies gain Traction for Cancer Diagnosis
Oct 04 2022
In search of a simple, non-invasive and economically feasible way of diagnosing cancer, an approach adopted by the Weizmann Institute of Science may lead to a blood test that will diagnose cancer with unprecedented accuracy.
“Many of the conventional methods clinically available today to detect and diagnose cancer are invasive and unpleasant,” explains research lead Dr. Efrat Shema of Weizmann’s Immunology and Regenerative Biology Department. “Eliminating the discomfort means that people would be less likely to avoid getting tested – and more likely to have their cancers detected earlier,” said Vadim Fedyuk, who led the study together with fellow graduate student Nir Erez.
The idea behind using liquid biopsies arose from the fact that blood contains free-floating DNA and proteins shed by dead blood cells in healthy people – and in cancer patients, by dead tumor cells as well. “Some of the byproducts of cell destruction, including cancer DNA and proteins, are dumped into the bloodstream and we know how to collect and analyse them,” Shema said.
Her approach to development of a test to assess multiple epigenetic parameters, builds on a method for imaging individual molecules that she had developed during her postdoctoral research at Harvard Medical School and the Broad Institute. The method makes it possible to achieve accurate epigenetic mapping with only a very small amount of raw material, using a fluorescent microscope. It can be employed, for example, to view epigenetic markings on nucleosomes, pieces of DNA wrapped around protein “spools.” These may be shed into the bloodstream like bits of flotsam when cells are destroyed, so Shema reasoned that the millions of nucleosomes found in the blood could be analysed to detect cancer.
Using Shema’s single-molecule imaging method, Fedyuk and Erez, together with colleagues, compared the nucleosomes in the blood of 30 healthy individuals with those of 60 patients with different stages of colorectal cancer. They found that the nucleosomes of the two groups were characterised by vastly different patterns of epigenetic marking. This analysis covered six different epigenetic modifications linked to cancer, as well as a variety of other cancer indicators, including protein segments from dead tumors, which are undetectable by conventional technologies.
Next, in collaboration with Prof. Guy Ron from the Racah Institute of Physics at the Hebrew University of Jerusalem, the scientists combined what they had revealed about the molecular biology of cancer with artificial intelligence algorithms, applying machine learning to the large data sets obtained from the two groups. The scientists also applied their technology to compare blood nucleosomes of healthy volunteers with those of 10 patients with pancreatic cancer.
“Our algorithm could tell the difference between the healthy and the patient groups at a record level of certainty for studies of this type – with 92 percent precision,” Shema says. The scientists call the new technology EPINUC, an acronym for “epigenetics of plasma-isolated nucleosomes.”
If supported by studies involving a greater number of patients, these findings could lead to a multiparameter blood test for detecting and diagnosing cancer using less than 1 ml of blood. In the future, because of the level of detail revealed in the analysis, the results of this blood test might also advance personalised medicine by suggesting the best treatments for each individual patient.
Shema sums up: “We’ve achieved a successful proof of concept for our method, which now needs to be confirmed in clinical trials. In the future, our multiparameter approach may serve to diagnose not only various cancers but also additional diseases that leave traces in the blood, such as autoimmune disorders or heart disease.”
Dr. Efrat Shema’s research is supported by the Swiss Society Institute for Cancer Prevention Research and the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics.
Dr. Shema is the incumbent of the Lisa and Jeff Aronin Family Career Development Chair.
The research was published in Nature Biotechnology.
More information online
Digital Edition
International Labmate 49.6 - Sept 2024
September 2024
Chromatography Articles - HPLC gradient validation using non-invasive flowmeters Mass Spectrometry & Spectroscopy Articles - From R&D to QC, making NMR accessible for everyone: Putting NMR...
View all digital editions
Events
Oct 30 2024 Birmingham, UK
Oct 30 2024 Manchester, UK
Nov 11 2024 Dusseldorf, Germany
Nov 12 2024 Cologne, Germany
Nov 12 2024 Tel Aviv, Israel


.jpg)















