Laboratory Products
Ames Software for ProtoCOL 3 Now Features Mutagenicity Ratio Functionality Offering Laboratories Fast, Reproducible Toxicology Testing Results
May 01 2019
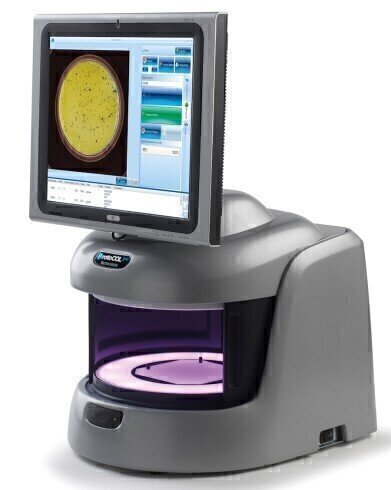
Synbiosis, a long-established, expert manufacturer of automated microbiological systems, today introduced its next generation Ames software module featuring a time-saving Ames mutagenicity ratio calculation function. The new software designed for integrated use with the ProtoCOL 3 colony counter, facilitates rapid production of accurate, consistent, count and ratio data from Ames test plates and is ideal for use in regulated toxicology testing laboratories.
The new Ames software enables ProtoCOL 3 to quickly count Salmonella typhimurium or E.coli colonies cultured on, for example, Vogel Bonner medium before and after exposure to potentially toxic or non-toxic control compounds. The software can then automatically compare the different colony counts to generate an Ames mutagenicity ratio, providing a quick guide to the mutagenic potential of a test chemical. This saves microbiologists time and effort with transferring count data to statistical software or manually calculating the ratio. It means ratio results are objective as they are not dependent on the scientist performing the tasks.
As well as numerical data, the software also produces full colour images of Ames test plates which can be stored in a secure SQL database for reference and another count, if required. Count and ratio data can be automatically downloaded to a spreadsheet (Excel/ OpenOffice) or LIMS, thus eliminating data transfer errors, to generate reproducible, traceable results, which are consistent from one microbiologist to another.
Featuring user access levels and a full audit trail with user login and logout records, the new Ames software is GLP compliant and can be used in a 21 CFR Part 11 environment. The archived results are suitable for generating high-quality reports for audit by regulatory authorities, making the new Ames software appropriate for use in pharmaceutical toxicology testing and other regulated microbiology laboratories.
Users with Ames software licences for ProtoCOL 3 can download the upgrade free of charge from here.
“Manually calculating the Ames mutagenicity ratio by comparing different sets of colony counts is a common approach for determining if a compound is mutagenic, but it is time consuming and can be prone to human error,” says Kate George, Sales and Technical Director at Synbiosis. “Our new module with its ratio calculation function is a time-saving solution to this problem and makes the ProtoCOL 3 and integrated Ames software an intelligent choice for any toxicology testing laboratory.”
Digital Edition
ILM 49.5 July
July 2024
Chromatography Articles - Understanding PFAS: Analysis and Implications Mass Spectrometry & Spectroscopy Articles - MS detection of Alzheimer’s blood-based biomarkers LIMS - Essent...
View all digital editions
Events
Jul 28 2024 San Diego, CA USA
Jul 30 2024 Jakarta, Indonesia
Jul 31 2024 Chengdu, China
ACS National Meeting - Fall 2024
Aug 18 2024 Denver, CO, USA
Aug 25 2024 Copenhagen, Denmark
-(1)-(1).jpg)


24_06.jpg)













