-
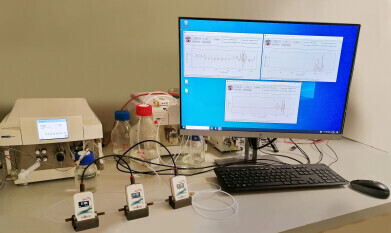 Acquisition and traceable logging of flow data from multiple different HPLC systems.
Acquisition and traceable logging of flow data from multiple different HPLC systems.
HPLC
Continuous validation of HPLC performance in regulated laboratories
Mar 07 2024
Testa Analytical announces the successful completion of extensive testing for its flowmeter software driver designed for chromatography data systems (CDS) in a regulated pharmaceutical laboratory environment.
Robust testing of new software packages and drivers is a crucial aspect of any product release, particularly when the software is intended to seamlessly interface with chromatography software packages from various HPLC and GPC/SEC manufacturers.
Jeanette Ziemba, Technical Manager at Testa Analytical, stated: “Our new flowmeter software driver, after comprehensive in-house testing, underwent extensive evaluation in a leading European pharmaceutical lab. Managing a diverse range of HPLC instruments with different chromatography data systems in a tightly regulated client-server environment, the lab was pleased with how the software driver facilitated easy acquisition and traceable logging of flow data from all its liquid chromatographs. The software's capability to support multiple Flowmeters connected to different HPLC systems in both analytical and semi-prep applications significantly streamlines tasks.”
She further explained: “The ability to save real-time flow rate data with each chromatogram is a valuable tool for comprehensive quality assessment of any HPLC, UHPLC, LC/MS, or GPC/SEC system. The software driver, built on rc.NET technology, ensures cross-platform connectivity of all Testa Analytical flowmeters to Chromatography Data System software packages, including OpenLab (Agilent Corp), Clarity Chromatography Software (DataApex), Empower (Waters Corp), LabSolutions (Shimadzu Corp), and WinGPC Software (Agilent).”
Ms Ziemba concluded: “The capability to collect and store real-time flow rate data, along with chromatography detector signals, not only enhances quality assessment but also provides continuous validation of compliance with the planned chromatographic separation method. In essence, this affordable flowmeter software driver complements and extends the capabilities of any modern liquid chromatography software package.”
More information online
Digital Edition
ILM 49.5 July
July 2024
Chromatography Articles - Understanding PFAS: Analysis and Implications Mass Spectrometry & Spectroscopy Articles - MS detection of Alzheimer’s blood-based biomarkers LIMS - Essent...
View all digital editions
Events
Jul 28 2024 San Diego, CA USA
Jul 30 2024 Jakarta, Indonesia
Jul 31 2024 Chengdu, China
ACS National Meeting - Fall 2024
Aug 18 2024 Denver, CO, USA
Aug 25 2024 Copenhagen, Denmark


24_06.jpg)













