Laboratory Products
Liquid Biopsy CDx Test for Advanced Non-small Cell Lung Cancer Receives FDA Approval
Dec 20 2022
The US Food and Drug Administration (FDA) has approved Agilent Resolution ctDx FIRST as a companion diagnostic (CDx) to identify advanced non-small cell lung cancer (NSCLC) patients with KRAS G12C mutations who may benefit from treatment with KRAZATITM (adagrasib).
The Agilent Resolution ctDx FIRST is said to offer NSCLC patients and their oncologists a new minimally invasive blood assay to help decide precision treatment options. This is the first liquid biopsy NGS assay approved by the FDA as a CDx for the newly approved KRAZATI in advanced NSCLC and was developed in collaboration with Mirati Therapeutics. ctDx FIRST has also been approved by the FDA for tumour profiling of the epidermal growth factor receptor (EGFR) gene for use by qualified healthcare professionals in accordance with professional guidelines in oncology patients with NSCLC.
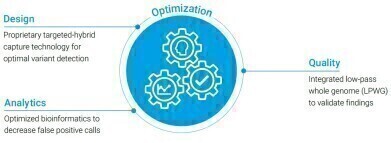
As a professional service, the ctDX FIRST test report includes broad genomic profiling on 109 genes across four types of alterations: single nucleotide variants (SNVs), insertions and deletions (indels), copy number amplifications (CNAs), and fusions.
The ctDx FIRST assay uses novel propriety technology to detect genomic alterations in circulating tumour DNA (ctDNA) from plasma.
“Expanding access to accurate and fast genomic profiling is an essential step to unlocking important medicines for patients in desperate need,” said Kenna Anderes, Mirati’s Vice President of Translational Medicine and Companion Diagnostics. “We appreciate the opportunity to partner with companies like Agilent who are committed to creating more opportunities for ‘decision medicine’ for people living with cancer.”
“We are thrilled to partner with Agilent as they work to create greater access to efficient, effective and minimally-invasive tests to support clinicians with information that is critical to their patient care,” said Alan Sandler, MD, Mirati’s Chief Medical Officer. “At Mirati, we are focused on creating meaningful impacts on the lives of people with cancer. Tests like ctDx FIRST are important to realising our commitment to patients.”
“Commercialising the ctDx FIRST test enables us to support clinicians to positively impact the lives of patients with advanced NSCLC,” said Sam Raha, Agilent’s President of the Diagnostics and Genomics Group. "Agilent values opportunities to partner with Mirati and other pharmaceutical companies in developing clinically relevant NGS-based diagnostics that enhance confidence in targeted cancer therapy."
More information online
Digital Edition
ILM 49.5 July
July 2024
Chromatography Articles - Understanding PFAS: Analysis and Implications Mass Spectrometry & Spectroscopy Articles - MS detection of Alzheimer’s blood-based biomarkers LIMS - Essent...
View all digital editions
Events
Jul 28 2024 San Diego, CA USA
Jul 30 2024 Jakarta, Indonesia
Jul 31 2024 Chengdu, China
ACS National Meeting - Fall 2024
Aug 18 2024 Denver, CO, USA
Aug 25 2024 Copenhagen, Denmark
-(1)-(1).jpg)


24_06.jpg)













