Laboratory Products
Fda Approval Received for Her2 Image Analysis Application
Apr 24 2008
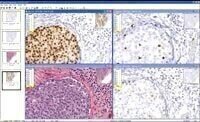
intended to be used as an aid to pathologists in detecting and quantifying HER2 protein expression from digital slide images created by Aperio’s slide scanning systems.
Aperio’s FDA clearance encompasses the company’s complete digital pathology system, including ScanScope® scanners for creating digital slide images from microscope slides, the Spectrum™ digital pathology information management system for managing, viewing, and analysing
digital slides, and the specific image analysis application which performs the automated scoring of IHC HER2 breast cancer digital slides.
Digital Edition
ILM 49.5 July
July 2024
Chromatography Articles - Understanding PFAS: Analysis and Implications Mass Spectrometry & Spectroscopy Articles - MS detection of Alzheimer’s blood-based biomarkers LIMS - Essent...
View all digital editions
Events
Jul 28 2024 San Diego, CA USA
Jul 30 2024 Jakarta, Indonesia
Jul 31 2024 Chengdu, China
ACS National Meeting - Fall 2024
Aug 18 2024 Denver, CO, USA
Aug 25 2024 Copenhagen, Denmark
-(1)-(1).jpg)


24_06.jpg)













