Laboratory Products
Instrument qualification - an essential step towards data integrity
Sep 01 2020
Failure to achieve data integrity is a significant cause of regulatory non-compliance: in 2018 almost half (49%) of the 85 Warning Notices issued on GMP by the FDA in 2018 cited data integrity deficiencies. Data Integrity is all about the reliability of data and as the data lifecycle usually begins with measured values, confirming the validity of those values is key to establishing overall data integrity. If that original data is generated by an instrument such as a spectrophotometer, then the instrument itself must be shown to be working correctly, by the process of Instrument Qualification.
UV-Visible spectrophotometry is one of the most widely used instrumental techniques in pharmaceutical analysis and the pharmacopoeias give guidance on the performance criteria required.
The USP describes four stages of Analytical Instrument Qualification (AIQ): design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
OQ is probably the most frequently performed and involves qualifying the instrument against standards having accurately known properties. A spectrophotometer must usually be qualified for wavelength, absorbance, stray light and resolution (spectral bandwidth). In the past a few generic tests could establish pharmacopoeia compliance but recent updates to the USP and EP now require users to demonstrate ‘fitness for purpose’, so qualification measurements must be made at parameter values matching those to be used for analysis. If a variety of analyses is to be carried out, a range of reference materials may be needed to cover the parameter values involved. Fortunately, suitable materials are commercially available as Certified Reference Materials (CRMs). Both EP and USP allow the use of CRMs, indeed the USP strongly advocates their use in preference to laboratory-prepared references.
Choosing wavelength CRMs is relatively straightforward as most of them have several certified peak values over their working ranges. Rare earth references such as holmium and didymium have been used for many years, but the new USP and EP regulations recommend cerium solutions for wavelengths below 240 nm. Absorbance CRMs require a little more thought. In the UV, between 210 and 250 nm, nicotinic acid is the usual recommendation.
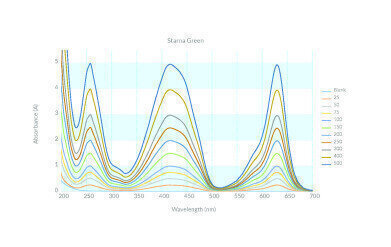
At higher UV wavelengths there is more choice: potassium dichromate solutions, the proprietary Starna Green CRM and metal-on-quartz filters. Starna Green has peaks over a wider wavelength range than potassium dichromate and can be certified up to 5 A so may prove more widely applicable.
The choice of CRM supplier is as important as the choice of CRM. A filter with a calibration certificate is not necessarily a CRM! According to ISO a fully accredited CRM supplier should be accredited to both ISO 17034:2016 and ISO/IEC 17025:2017 and a proposed supplier’s Schedule of Accreditation should be checked to confirm that it includes the reference material to be purchased – it may not! Instrument qualification using CRMs from a properly accredited supplier is the first step towards data integrity.
Digital Edition
International Labmate 49.6 - Sept 2024
September 2024
Chromatography Articles - HPLC gradient validation using non-invasive flowmeters Mass Spectrometry & Spectroscopy Articles - From R&D to QC, making NMR accessible for everyone: Putting NMR...
View all digital editions
Events
Oct 30 2024 Birmingham, UK
Oct 30 2024 Manchester, UK
Nov 11 2024 Dusseldorf, Germany
Nov 12 2024 Cologne, Germany
Nov 12 2024 Tel Aviv, Israel