-

-
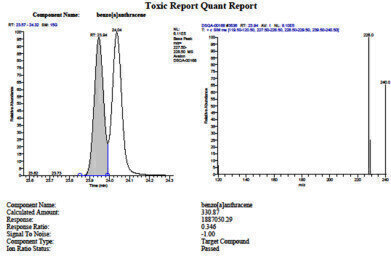 Figure 1. Ion spectra for benzo(a)-anthracene from analysis of NIST 1944, showing resolution from chrysene.
Figure 1. Ion spectra for benzo(a)-anthracene from analysis of NIST 1944, showing resolution from chrysene. -
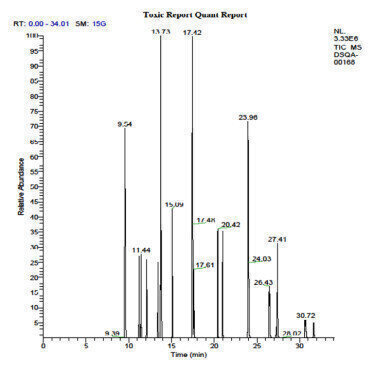 Figure 2. Total ion chromatogram from NIST 1944 analysis.
Figure 2. Total ion chromatogram from NIST 1944 analysis.
Chromatography
Method for the Validation of PAHs in Soil and Sediment Samples Using Pressurized Liquid Extraction and Automated Cleanup
Aug 07 2013
Introduction
PAHs are a group of organic compounds consisting of two or more benzene rings and are often the byproduct of petroleum combustion. Due to their carcinogenic characteristics at relatively low concentra-tions, they are of particular environmental concern. Seven PAHs have been classified by the US EPA as probable human carcinogens and their toxic characteristics and persistent nature place them among the most extensively monitored organic contaminants.
The following procedure details the use of Pressurized Liquid Extraction (PLE) in conjunction with automated silica gel cleanup (PowerPrep) to deliver a robust, efficient sample prep process for soils and sediments designated for PAH analysis. Outlined, are an Initial Precision and Recovery Study (IPR), a Matrix Detection Level Study (MDL) and a matrix validation of a NIST reference material.
Instrumentation and Consumables
- FMS, Inc. PLE System
- FMS, Inc. PowerPrep System
- FMS, Inc. SuperVap Concentrator System
- FMS, Inc. 50 mL direct-to-vial concentrator tubes
- FMS, Inc. 200 mL concentrator tubes (1 mL termination)
- Thermo Scientific Trace Ultra GC with DSQ MS
Consumables
- FMS, Inc. 6 gram neutral silica columns
- Fisher Pesticide Optima* n-Hexane
- Fisher Pesticide Optima* Methylene Chloride
- Agilent Hydromatrix©
- NIST 1944 RM; NJ River Sediment
- Restek PAH Mixture (Cat# 31841)
- Restek Surrogate Mixture (Cat# 31062)
- Restek SV Internal Standard Mixture (Cat# 31006)
Procedure:
Sample Prep
Samples are weighed out in glass beakers.
For IPR and MDL samples, 20 grams of baked Ottawa sand was used.
Sample portions are spiked with surrogate solutions and/or PAH spiking solution.
Samples are generously mixed with Hydromatrix.
Dried samples are transferred to 40 mL PLE extractions cells.
Pressurized Liquid Extraction System
- Cells filled with hexane: DCM (50:50)
- Cells pressurized to 1500 PSI
- Cells heated to 120 ºC for 20 minutes
- Cells cooled to ambient temperature
- Cells flushed with 20 mL solvent
Cells purged with N2 and extract discharged to SuperVap Concentrator
SuperVap Concentration System
Preheat temp: 20 minutes at 60 °C
Evaporation mode w/Sensor temp: 60 °C
Nitrogen Pressure: 10 PSI
PowerPrep System
- Condition column(s) w/10 mL DCM
- Exchange column(s) to Hexane
- Load sample extract(s)
- Flush column(s) w/10 mL hexane
- Elute column(s) w/35 mL DCM
- Extract eluted to 50 mL SuperVap concentrator tubes with Direct-to-GC vial connection.
Results
Table 1. Results of four replicate IPR study (spiked at 500 µg/kg)
| Mean | STD | |
| Compound | Rec. | DEV |
| Naphthalene | 85.10% | 2.10% |
| 2-Methylnaphthalene | 91.50% | 2.00% |
| 1-Methylnaphthalene | 88.90% | 2.10% |
| Acenaphthylene | 101.50% | 1.90% |
| Acenaphthene | 96.50% | 2.50% |
| Fluorene | 96.90% | 3.30% |
| Phenanthrene | 89.10% | 4.60% |
| Anthracene | 116.90% | 4.50% |
| Fluoranthene | 102.60% | 5.90% |
| Pyrene | 101.10% | 5.60% |
| Benzo[a]anthracene | 97.40% | 4.60% |
| Chrysene | 104.70% | 5.10% |
| Benzo[b]fluoranthene | 90.00% | 7.10% |
| Benzo[k]fluoranthene | 95.20% | 3.70% |
| Benzo[a]pyrene | 89.50% | 3.70% |
| Indeno[1,2,3-cd]pyrene | 82.00% | 4.70% |
| Dibenzo[a,h]anthracene | 78.70% | 4.50% |
| Benzo[g,h,i]perylene | 83.30% | 4.40% |
| Nitrobenzene-D5 (Surr) | 93.60% | 6.10% |
| 2-Fluorobiphenyl (Surr) | 80.20% | 3.00% |
| p-Terphenyl-d14 (surr) | 81.70% | 5.20% |
Table 2. Results of NIST 1944 analysis (reported in mg/kg)
| Cert. | Calc. | |
| Compound | Value | Value |
| Naphthalene | 1.28 | 0.986 |
| 2-Methylnaphthalene | 0.74 | 0.589 |
| 1-Methylnaphthalene | 0.47 | 0.356 |
| Acenaphthylene | NA | 0.631 |
| Acenaphthene | 0.39 | 0.363 |
| Fluorene | 0.48 | 0.371 |
| Phenanthrene | 5.27 | 4.06 |
| Anthracene | 1.13 | 1.51 |
| Fluoranthene | 8.92 | 7.55 |
| Pyrene | 9.7 | 7.58 |
| Benzo[a]anthracene | 4.72 | 3.44 |
| Chrysene | 4.86 | 4.01 |
| Benzo[b]fluoranthene | 3.87 | 3.41 |
| Benzo[k]fluoranthene | 2.3 | 1.83 |
| Benzo[a]pyrene | 4.3 | 3.12 |
| Indeno[1,2,3-cd]pyrene | 2.78 | 2.26 |
| Dibenzo[a,h]anthracene | 0.42 | 0.445 |
| Benzo[g,h,i]perylene | 2.84 | 2.31 |
Table 3. Results of 7 replicate MDL study (spiked at 10 µg/kg)
| MDL | STD | |
| Compound | µg/kg | DEV |
| Naphthalene | 2.57 | 0.815 |
| 2-Methylnaphthalene | 2.82 | 0.905 |
| 1-Methylnaphthalene | 2.83 | 0.9 |
| Acenaphthylene | 2.93 | 0.93 |
| Acenaphthene | 3.4 | 1.08 |
| Fluorene | 1.19 | 0.38 |
| Phenanthrene | 3.38 | 1.08 |
| Anthracene | 2.83 | 0.9 |
| Fluoranthene | 2.68 | 0.85 |
| Pyrene | 2.22 | 0.705 |
| Benzo[a]anthracene | 3.96 | 1.26 |
| Chrysene | 4.89 | 1.56 |
| Benzo[b]fluoranthene | 1.97 | 0.625 |
| Benzo[k]fluoranthene | 3.22 | 1.03 |
| Benzo[a]pyrene | 1.78 | 0.565 |
| Indeno[1,2,3-cd]pyrene | 1.68 | 0.535 |
| Dibenzo[a,h]anthracene | 3.45 | 1.1 |
| Benzo[g,h,i]perylene | 4.63 | 1.47 |
Conclusions
Following the extraction and cleanup with silica gel, the extracts were analyzed on a Thermo Scientific Trace GC with DSQ Mass Spectrometer. All analysis was performed in the selective ionization mode (SIM), with one quantitation ion and one confirmation ion monitored.
Figure 1. Ion spectra for benzo(a)-anthracene from analysis of NIST 1944, showing resolution from chrysene.
Analysis of the extracts showed excel-lent extraction efficiencies for all compounds analyzed, with minimal deviation between runs. The high level of efficiency enabled the establishment of an MDL below the target of 5 µg/kg for all analytes using the designated sample size. The calculated concentrations for the NIST 1944 sample were between 70-130% for all analytes, thus validating the PLE system extraction for soil and sediment sample. Due to the wide array of other organic contaminants present in the NIST reference sample, the efficiency of the 6 gram silica column was further validated by the clear resolution of each target analyte.
Figure 2. Total ion chromatogram from NIST 1944 analysis.
Using the FMS Pressurized Liquid Extraction system in conjunction with the PowerPrep Sample Cleanup system demonstrates an efficient and robust sample prep method that delivers both high quality results and increased sample throughput. By combining extraction, concentration and cleanup with direct-to-GC-vial concentration, this automated sample-to-vial process frees laboratory staff to perform other tasks which increases the lab’s throughput and quality and consistency of results.
For more information contact FMS at:
FMS Inc. Email: onlineinfo@fms-inc.com or Web site: fmsenvironmental.com
Digital Edition
ILM 49.5 July
July 2024
Chromatography Articles - Understanding PFAS: Analysis and Implications Mass Spectrometry & Spectroscopy Articles - MS detection of Alzheimer’s blood-based biomarkers LIMS - Essent...
View all digital editions
Events
Jul 28 2024 San Diego, CA USA
Jul 30 2024 Jakarta, Indonesia
Jul 31 2024 Chengdu, China
ACS National Meeting - Fall 2024
Aug 18 2024 Denver, CO, USA
Aug 25 2024 Copenhagen, Denmark



24_06.jpg)













