IT Solutions
Data Integrity for Challenging Regulatory Environments
Feb 24 2021
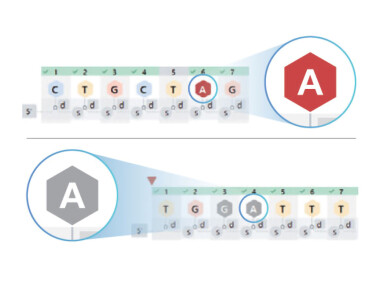
LabWare continues to deploy solutions that meet the challenging regulatory requirements for all the industries they support. The company are constantly evolving to ensure they meet the future needs in regulatory environments that continue to become more data- and system-centric.
In association with a customer’s procedures and quality systems, LabWare offers a configurable solution that can provide a compliant and validated environment for the functions used. With comprehensive auditing, review functionality, and system controls, these implementations have been proven to meet the demands of agencies such as the FDA, MHRA, and WHO in GMP and GLP laboratories.
More information online
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK