IT Solutions
How Secure are Your UV/Vis Electronic Records? Ensure Data Integrity Compliance with these 7 Solutions
Oct 03 2018
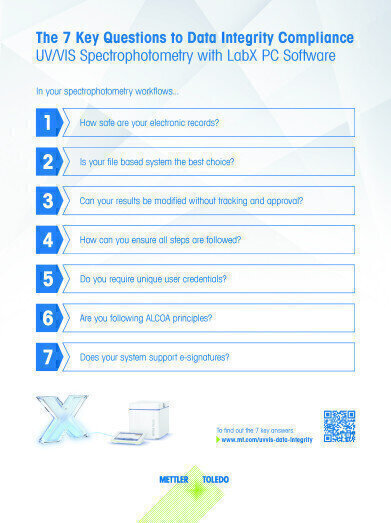
Are you worried about FDA warning letters? In 2016, 80% of the FDA warning letters issued were due to lack of data integrity. The primary reason was incomplete data. This problem can be prevented with the right solutions.
The completeness, security and accuracy of your UV/VIS electronic records are essential in fulfilling the FDA 21 CFR Part 11 guidelines concerning data integrity. A combination of using efficient, secure software to store your records, along with implementing Good UV/VIS Practice removes the risk of such warning letters. METTLER TOLEDO offers a comprehensive solution to all elements of these guidelines in the form of LabX PC software. By following sample analysis SOPs, providing secure SQL database storage, supporting e-signatures, and requesting user authentication for authorized data access, LabX streamlines the process of compliance into one straightforward solution.
Does your current UV/VIS system comply with data integrity requirements? Download our infographic to get the 7 key answers to data integrity for UV/VIS workflows. These topics form the foundation for a well-rounded and compliant solution to achieve optimal data integrity.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK
May 21 2024 Lagos, Nigeria











.jpg)





