Laboratory Products
Breakthrough 25-minute COVID-19 Test set for Launch
Oct 21 2020
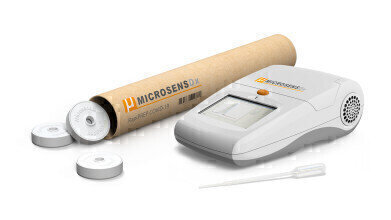
A breakthrough test for COVID-19 which works in just 25 minutes, with high accuracy and requiring only routine laboratory equipment, is currently in the final stages of clinical validation by British biotech firm MicrosensDx.
The test, called MicrosensDx COVID-19, has been used successfully in large scale testing programs with BAE Systems in Cumbria over the last few months, where it has been deployed to test more than 8,000 people a week. MicrosensDx is now planning to scale up for wider availability, both in the UK and across Europe.
Crucially, the test uses fewer of the globally limited reagents that other COVID-19 test technologies require and which have been in short supply across Europe in recent months.
It can be used within mass testing laboratories, but is also available for use in a ‘near patient’ mini-lab format, by healthcare staff with basic laboratory training, to enable rapid real-time testing. Crucially the result is available in less than a third of the time required by the widespread Public Health England PCR test [90 minutes].
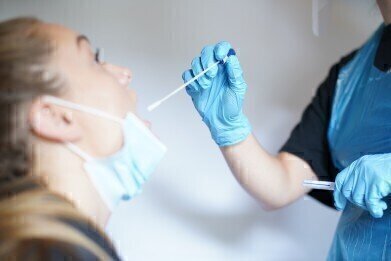
The test can analyse multiple types of sample (throat and nasal swabs, in addition to saliva and sputum) and, crucially, the virus is neutralised once it is captured in a vial, making it safe for the operator administering the test. The sample is placed in a solution containing the company’s highly-sensitive magnetic beads which attract RNA from the virus, even at the lowest of levels. This allows the RNA to be extracted from the patient’s sample ready for amplification.
The detection technology is based on the biotech’s loop-mediated isothermal amplification (LAMP) technology.
MicrosensDx COVID-19 is a refined version of a prototype test which was originally designed earlier this year and validated in collaboration with Kings College London’s Professor Tim Spector OBE, the world-leading epidemiologist. The sensitivity of the test has improved markedly since the early-stage assay, with validation data due later this month to coincide with full CE marking accreditation.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK



.jpg)














