Laboratory Products
Slides that Run 2 Diagnostics Tests Simultaneously Awarded CE Mark
May 10 2019
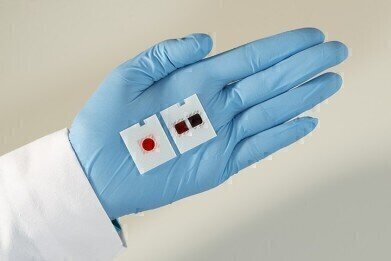
Ortho Clinical Diagnostics’ new VITROS® XT MicroSlide system has been awarded CE Mark certification. This groundbreaking, multi-test technology allows labs to run two tests on one slide simultaneously, thereby simplifying workflow and improving productivity and turnaround time in the lab. Currently, available test pairs include: triglycerides and cholesterol; urea and creatinine; glucose and calcium; bilirubin and alkaline phosphatase.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK