Laboratory Products
CytoQuant® - Quantify bacteria and residues in just 30 seconds!
May 10 2022
Cracking down on bacteria
The presence of bacteria in food and beverage manufacturing facilities can affect product shelf life and quality, while pathogens can even lead to foodborne illness. Cleaning and disinfection is therefore paramount to securing food safety and protecting both your reputation and your business. But cracking down on something we can’t see isn’t always easy and straightforward. While visual inspection is a good first step, it isn‘t enough. Traditional microbiological methods often don’t allow for preventive control and preoperational actions as lab results take days. ATP tests, while simple and fast, quantify biological residues, which are not a meaningful proxy for disinfection efficacy. Each one of these methods comes with considerable limitations. Yet you are expected to make informed decisions, fast.
Care to learn more? Click here to book a free demo!
How does CytoQuant® work?
By using impedance flow cytometry, the CytoQuant® device takes advantage of the unique electromagnetic properties of the structure of bacteria (cell membrane and cytoplasm) to distinguish them from other particles. Find out more about this technology through this video.
What exactly do we measure?
CytoQuant® provides precise counts of intact cells and particles. Intact cells are defined as cells with an intact cell wall, irrespective of their state (such as stressed, or viable but not culturable) or required growth conditions (aerobic or anaerobic, pH, nutrition, salt concentration, temperature, lag-time, incubation time, etc.). Because no solid or liquid media can cover this full range, and because only about 1% of existing bacteria are culturable (Staley & Konopka, 1985; Amann et al., 1995; Hugenholtz et al., 1998), intact cell counts and plate counts are two very different parameters. CytoQuant® additionally classifies any objects that are not bacteria and pass through the microfluidic flow cell as residue particles. Particle counts are a direct indication of cleanliness
How reliable are CytoQuant® intact cell count results?
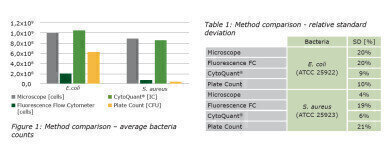
The accuracy of CytoQuant® measurements has been the subject of a large number of studies. In the latest, described below, Romer Labs partnered with a university and compared the intact cell counts of the CytoQuant® mobile flow cytometer with counts from a fluorescence microscope, a fluorescence flow cytometer and those from aerobic plates (TSA: tryptic soy agar). Overnight cultures were prepared from pure cell cultures with a known cell count. Dilutions were made to cover the whole quantification range of both flow cytometers (104-107 cells/mL). Measurements were done in triplicate. Microscope and plate counts were conducted at about 106 cells/mL and about 100 cfu/plate, respectively.
Not only were counts from CytoQuant® closest to those of the reference microscope method (Figure 1), CytoQuant® also had the lowest relative average standard deviations of all methods (Table 1). As expected, TSA plates returned the lowest bacteria counts as not all colonies emerge from a single cell and not all cells eventually multiply and form colonies. The fluorescence flow cytometer had significantly lower bacteria counts than CytoQuant® and the reference microscope method.
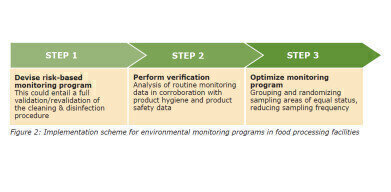
Implementing CytoQuant®
The implementation of CytoQuant® is not essentially different from that of other, better-known environmental monitoring methods, such as culturing or ATP testing. What makes CytoQuant® different is that it empowers you to verify your cleaning and disinfection procedure on the spot by allowing you to create preventive control strategies. Implementation tactics usually involve a three-step plan and depend on whether a validated cleaning and disinfection procedure is already in place.
Understand how CytoQuant® can value-add to your current environmental monitoring scheme, Book a free demo today!
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK
-012.jpg)


.jpg)














