-
 Digital technology ensures the accuracy of patient identification and streamlines processes.
Digital technology ensures the accuracy of patient identification and streamlines processes. -
 Fine Cut Group are UL Certified which means the materials used have been tested and pass the industry-wide safety standards.
Fine Cut Group are UL Certified which means the materials used have been tested and pass the industry-wide safety standards. -
Laboratory Products
The Future of Laboratory Labelling
Apr 21 2022
According to market research carried out by FMI (Future Market Insights) the global healthcare and laboratory labels market stands at over £7 billion as of this year and is expected to be worth over £10 billion by the year 2030. The rising need for improved healthcare has led to the development of new drugs, and clinical research is set to play a prominent role in the growth of the market over the next eight years.
Fine Cut Group have spoken countless times about the use of manual, handwritten labelling solutions - these methods are prone to errors and durability issues causing illegibility and inaccuracy of patient identification and overall visibility. The use of technology within the healthcare and laboratory labels market is a way forward to easily eliminate these issues.
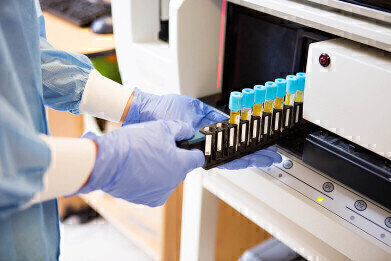
By using digital technology, such as, scannable 1D & 2D barcodes - QR codes and Data Matrix, combined with human readable data - the accuracy of patient identification and streamlining processes can be enhanced by transmitting the data in real time to those who need it, delivering critical time saving and much needed accuracy.
The COVID-19 Pandemic has hugely impacted the way consumers interact with products, shifting the focus towards the use of technology and contact-less healthcare. FMI suggests that digital integration in healthcare and laboratory labels is anticipated to boost contact-less healthcare. Laboratory labelling has been essential as part of the COVID-19 testing scheme, and the data collected from infected patients is very important for the ongoing and future research on the virus. COVID-19 alone has been a huge factor of the growth in the market due to the demand for labels and their data across the world.
Regulations from government bodies ensuring compliance is met for the standards of healthcare and laboratory labelling is ever changing and can prove challenging within the industry. Fine Cut Group are UL Certified which means the materials used have been tested and pass the industry-wide safety standards set by UL. Why does this matter? Peace of mind. All products can be purchased with the knowledge that they are verified and meet specific safety standards set by a third-party certification company.
As well as regulation changes, the ever-changing end user requirements such as sizing, printing process and materials means Fine Cut Group have to keep on top of their offerings to meet demand - nearly 65% of the healthcare label market is accounted for by paper-based labels, but Polyolefin-based labels (a type of polymer) - popular for their strong adhesive and durability, especially against hazardous chemicals, are expected to boost the market by around £11 million between now and 2030 according to FMI.
Along with paper and polymer, Fine Cut Group offer a large range of material and adhesive combinations to ensure they are able to meet the demand of clients and these ever-changing needs in supply. And lastly, the company offer a completely bespoke service, tailoring to the needs of each individual enquiry. With Fine Cut Group experts on hand, the best suited product for your requirement is guaranteed.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK

















