Laboratory Products
Expert Help Available for the Implementation of ISO 9001:2015 Revisions
Aug 22 2017
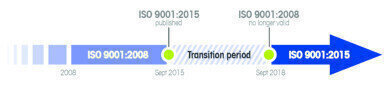
Changes to ISO 9001 were made in 2015. All ISO 9001 certified organisations have a 3 year transition period to update their quality management systems accordingly. If your processes involve any weighing steps, Mettler Toledo can support you to easily make the necessary updates, using the proven risk-based Good Weighing Practice™ approach. Do you need help to be ready by September 2018?
ISO 9001 is a standard that sets out the requirements for a Quality Management System (QMS). Its aim is to help businesses and organisations be more efficient and improve customer satisfaction. ISO 9001:2015 was released in 2015 and certified organisations have to adapt their processes to meet these changes by the September 2018 deadline. Greater emphasis is placed on the process approach, and risk-based thinking is now an important focus. Continuous improvement can be achieved by implementing the recommended Plan-Do-Check-Act (PDCA) cycle. But how do these elements relate to weighing?
Weighing has a critical impact on product quality. Quality is directly related to the accuracy of weighing results, and that accuracy is determined through calibration. A company is obliged to ensure that every balance or scale used in the weighing process is accurate enough to achieve the intended results.
To adopt the latest revisions to ISO 9001 for any processes which involve weighing, Mettler Toledo can support you to make the transition straightforward. Proven and well-established Mettler Toledo expertise, Good Weighing Practice™, was founded on the risk-based thinking approach. The elements which need to be considered for the PDCA cycle are already defined and fully developed within the GWP® Recommendation and GWP® Verification packages, making it easy to ensure that any weighing processes are ISO 9001 compliant.
Mettler Toledo’s ISO 9001 library collection for resources and support to learn more about this topic. Content such as white papers and on-demand webinars are available for download.
These free resources are designed to help you understand the scope of the ISO 9001:2015 changes, explain the implications on weighing processes, and provide guidance and recommendations on how to implement risk-based thinking with respect to weighing.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK


.jpg)














