-

-
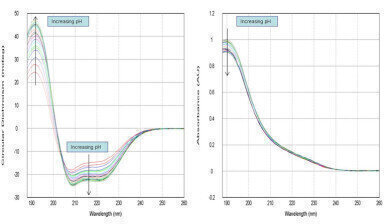 Figure 1
Figure 1 -
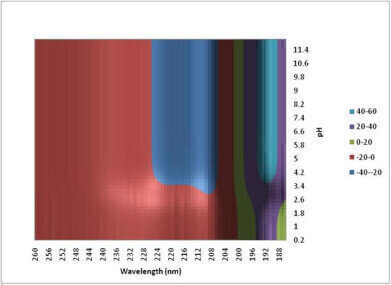 Figure 2.
Figure 2.
Chromatography
Mapping protein conformational stability using automated CD
Apr 06 2011
Applied Photophysics has automated sample preparation and transfer for its world-beating CD spectrometer, the ChirascanTM-plus. We have developed protocols that enable unattended running of up to 384 samples with washing, drying and measuring cycles that virtually eliminate sample dilution and cross contamination, offering the opportunity to study relative protein stabilities with ease. Typical studies include the influence of pH, ionic strength, detergent, mutation and ligand binding on protein conformation, important in biotherapeutic development, formulation testing and clone selection procedures.
With the growing interest in protein biotherapeutics as future drug entities, the importance of measuring protein structure and conformation in development and formulation is becoming of increasing importance. If a protein is not correctly folded, it will not execute its function properly, may aggregate and may even be immunogenic. CD spectroscopy is a sensitive probe for measuring protein conformation and Chirascan™plus ACD now offers considerable improvements including:
• sequentially delivering samples to the spectrometer cuvette from a microtitre plate with effectively zero inter-sample carry-over
• automatically acquiring and storing spectroscopic data for up to 384 (4 x 96-well plate) samples without operator intervention
• recovering sample to minimize waste
• simultaneously measuring more than one optical property (e.g. CD and absorption) to maximize the information return per sample
• interfacing seamlessly with data reduction programs designed to simplify the interpretation of spectroscopic data
• automated identification of spectrometer cuvette, setting protocols accordingly
In a recent study, Mapping protein stability using automated CD the robustness of the technique and data quality is demonstrated.
Figure 1: Solutions of BSA were studied in different pH environments, from pH 2.2 to pH 8. Buffer subtracted CD and absorption spectra shows the quality of automated data and changes in protein confirmation (260nm ≥ λ ≥ 188nm)
Figure 2: Experimental data can be used to generate a conformational stability map and in this way it is possible to get a quick semi-quantitative feel for the stability of the protein. The apparent loss of structure below pH 4.2 suggests that the protein is changing conformation.
The automated sample preparation and measurement of CD now enables experiments to be undertaken that were previously thought to be too time consuming or laborious, with applications such as pH and ionic strength titrations, protein stability experiments and biocomparabiltiy studies now very much in the domain of Circular Dichroism.
Find out more, contact us at:
www.photophysics.com, USA 1-800 543 4130, EU +44 (0) 1372 386537
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK
May 21 2024 Lagos, Nigeria
May 22 2024 Basel, Switzerland
Scientific Laboratory Show & Conference 2024
May 22 2024 Nottingham, UK






.jpg)










