Laboratory Products
Endotoxin Detection Portfolio Extended
Feb 07 2013
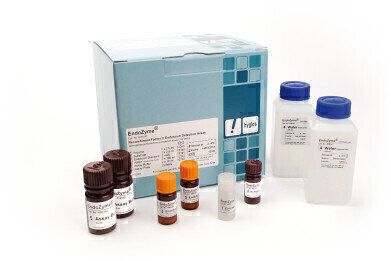
Hyglos has announced the commercial launch of EndoZyme®, a homogeneous fluorescence microplate assay using recombinant Factor C derived from horseshoe crab, for measuring endotoxin (Lipopolysaccharides, LPS) in pharmaceuticals, biologics and medical equipment.
Taking up on the pioneering work published by the Japanese scientists around Professor Sadaaki Iwanaga, the test developers at Hyglos have been able to finalise an improved recombinant Factor C (rFC) assay.
Recombinant Factor C, which is the essential part of EndoZyme®, is the endotoxin-specific principal receptor in the LAL enzyme cascade. In the assay recombinant Factor C is activated by any endotoxin present in the sample, recombinant Factor C enzymatically cleaves a synthetic substrate resulting in a fluorescence signal.
Lipopolysaccharides (LPS), or endotoxins, are biologically active components (toxins) of the outer cell membrane of all Gram-negative bacteria. Presence of endotoxins in the blood stream causes a triggering of the signalling cascade and may lead to endotoxic shock. In production of parental drugs, infusions and certain medical devices it is mandatory to control endotoxin.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
May 21 2024 Lagos, Nigeria
May 22 2024 Basel, Switzerland
Scientific Laboratory Show & Conference 2024
May 22 2024 Nottingham, UK
May 23 2024 Beijing, China
May 28 2024 Tel Aviv, Israel











.jpg)




