Laboratory Products
Biological Decontamination of Newly Constructed Hospital’s Clinical Areas Completed
Jun 04 2018
Bioquell, a leading global expert in reducing the risk of bio-contamination in the healthcare, life science and pharmaceutical markets, recently completed a 6-log decontamination of the key clinical areas within a newly constructed world class hospital, Sidra Medicine. The disinfection of 450 rooms totalling 56,000m3 was implemented in a short 15-day time frame ahead of the Qatar-based facility’s grand opening.
Bioquell performed a 6-log decontamination of a suite of 12 operating theatres, sterile processing unit, paediatric and neonatal intensive care units and other high risk patient areas such as oncology and dialysis. Working alongside numerous departments at Sidra Medicine, the project was successfully completed within the allocated three-week time frame.
The process was validated with Bioquell’s 6-log Geobacillus stearothermophilus biological indicators as well as a third party air sampling procedure organised by Sidra Medicine. A 6-log reduction was achieved in all areas within the scope with 100% of the independent air sampling results being within the acceptable range.
Bioquell’s Rapid Bio-decontamination Service (RBDS) was chosen to establish a degree of sterility as high as practically possible within key areas. The company used its scientifically-proven Hydrogen Peroxide Vapour technology due to its ability to offer a scalable solution while maintaining a high efficacy standard.
The larger areas being targeted, such as intensive care units and the hospital’s operating theatre complex, were treated as single, discrete decontamination zones, which minimised the risk of cross-contamination between rooms and corridors, and allowed the project to be completed in time.
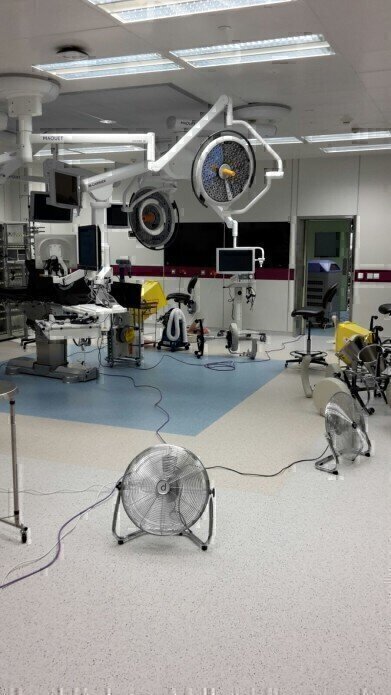
The largest area of the hospital to be treated as a single zone was the operating theatre complex with a total volume of c.7000m3. This entire suite was decontaminated within one day using 32 of Bioquell’s Hydrogen Peroxide Vapour generators simultaneously to provide effective decontamination. Distribution fans were also used to disperse the vapour within specified zones, whilst Bioquell’s aeration units provided the capacity to quickly break down the vapour to complete the decontamination cycle rapidly.
Bioquell’s 6-log Geobacillus stearothermophilus spore biological indicators were placed in challenging locations within each area, as well as proprietary Bioquell chemical indicators designed to give a real-time verification of a successful bio-decontamination process. Biological indicators provide insight to the success of a cycle after a 24-hour incubation period, with final results available after seven days.
All areas decontaminated strictly adhered to Bioquell safety protocols with doors giving access to each target area sealed using Bioquell tape to prevent leaks, warning signs placed to prevent entry to the area during the cycle and calibrated low level sensors to continually monitor the perimeter of each area throughout the cycle. The sensors also ensure that the Hydrogen Peroxide Vapour concentration had fallen below the safety limits after the cycle was complete.
Bioquell used 800 biological indicators to confirm the efficacy of the process. Additionally, Sidra Medicine also arranged for third party air sampling tests in all of the decontaminated areas. All the air sampling results were within the acceptable limits.
As demonstrated by the full inactivation of biological indicators and successful air sampling results, the project was completed safely and within the required time.
Following the successful delivery of the project, Sidra Medicine purchased multiple Bioquell BQ-50 Hydrogen Peroxide Vapour systems to perform regular decontamination cycles of single rooms and other small areas within the hospital.
Russell Gates, Office of the Vice Chairperson at Sidra Medicine commented: “The Bioquell team worked well with our own people and were very flexible in their approach. The planning prior to their arrival in Doha made the implementation of the project much easier as we were able to ensure the right people were in place to support the process.
“Our infection control team were very pleased with the results, especially as we were able to get initial indications within 24 hours. We are now looking forward to having our own staff trained on the Bioquell BQ-50 machines that we have purchased.”
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 25 2024 Istanbul, Turkey
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
.jpg)
















