-
 InMotion™ Karl Fischer Autosampler - Precise and Efficient Karl Fischer Titration Automation
InMotion™ Karl Fischer Autosampler - Precise and Efficient Karl Fischer Titration Automation -
 LabX Laboratory Software - Network Your Lab for Efficient, Compliant and Seamless Processes
LabX Laboratory Software - Network Your Lab for Efficient, Compliant and Seamless Processes -
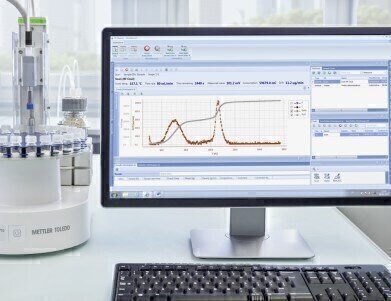 The Temperature Scanning Function in the InMotion™ KF Oven Autosampler
The Temperature Scanning Function in the InMotion™ KF Oven Autosampler
Laboratory Products
Residual Moisture in Lyophilized Vaccines - Boost Testing Productivity with Efficient Workflow
Jan 05 2021
Freeze-drying, also known as lyophilization is used in biological applications and food processing. The residual moisture content in lyophilized products plays a significant role in assessing both stability and shelf life. With vaccines, for example, the ability to determine the residual moisture content with acceptable accuracy and safety is crucial. For most products, the level of residual moisture should be low but not too low, as higher moisture levels often correlate with a decrease in stability. A residual moisture that is too low may cause aggregation or even influence further processing steps. With an increasing number of samples to analyse, scientists are under high time pressure to deliver results, while maintaining accuracy and safety. METTLER TOLEDO coulometric Karl Fischer titrators offer accurate and precise water determination with efficient workflows and traceable data that improves safety and productivity.
Reduce sample preparation steps, enhance sample throughput and accuracy of results
METTLER TOLEDO coulometric KF titrators and InMotion KF Oven Autosamplers directly determine the actual water content of lyophilized products in up to 120 closed vials per series. With an oven, the sample is heated, and the moisture is driven into the titrator via carrier gas so that the sample never directly contacts the KF reagent. Because only the water is titrated, side reactions are eliminated. The Temperature scanning function in the InMotion KF Oven Autosampler can be used to select a suitable oven temperature for the sample.
Ensure data traceability and integrity
One common software platform for METTLER TOLEDO balances and Karl Fischer titrators ensures data integrity and delivers support for FDA 21 CFR part 11. LabX Software collects the weighing data of your samples, and automatically transfers data from both balance and titrator, with combined reporting. Transcription and calculation errors are eliminated, making titration workflows more straightforward.
For example, consider the application of an automated water content determination of hygroscopic lyophilizates. The application describes a method to measure the actual water content of the lyophilized drugs in a closed vial. The gas phase extraction principle is used throughout the whole measuring procedure. The absolute water content is determined by an InMotion KF autosampler and the unknown sample weight is measured with LabX, based on the principle of backweighing. In order to fulfill USP <921>, and to prevent the sample from oxidation, nitrogen is used as an inert carrier gas.
Download the free application note, plus the LabX balance and titrator methods here.
Digital Edition
Lab Asia 31.2 April 2024
April 2024
In This Edition Chromatography Articles - Approaches to troubleshooting an SPE method for the analysis of oligonucleotides (pt i) - High-precision liquid flow processes demand full fluidic c...
View all digital editions
Events
Apr 28 2024 Montreal, Quebec, Canada
May 05 2024 Seville, Spain
InformEx Zone at CPhl North America
May 07 2024 Pennsylvania, PA, USA
May 14 2024 Oklahoma City, OK, USA
May 15 2024 Birmingham, UK


.jpg)














